Anion: Definition, Examples, Properties, and Real-Life Importance
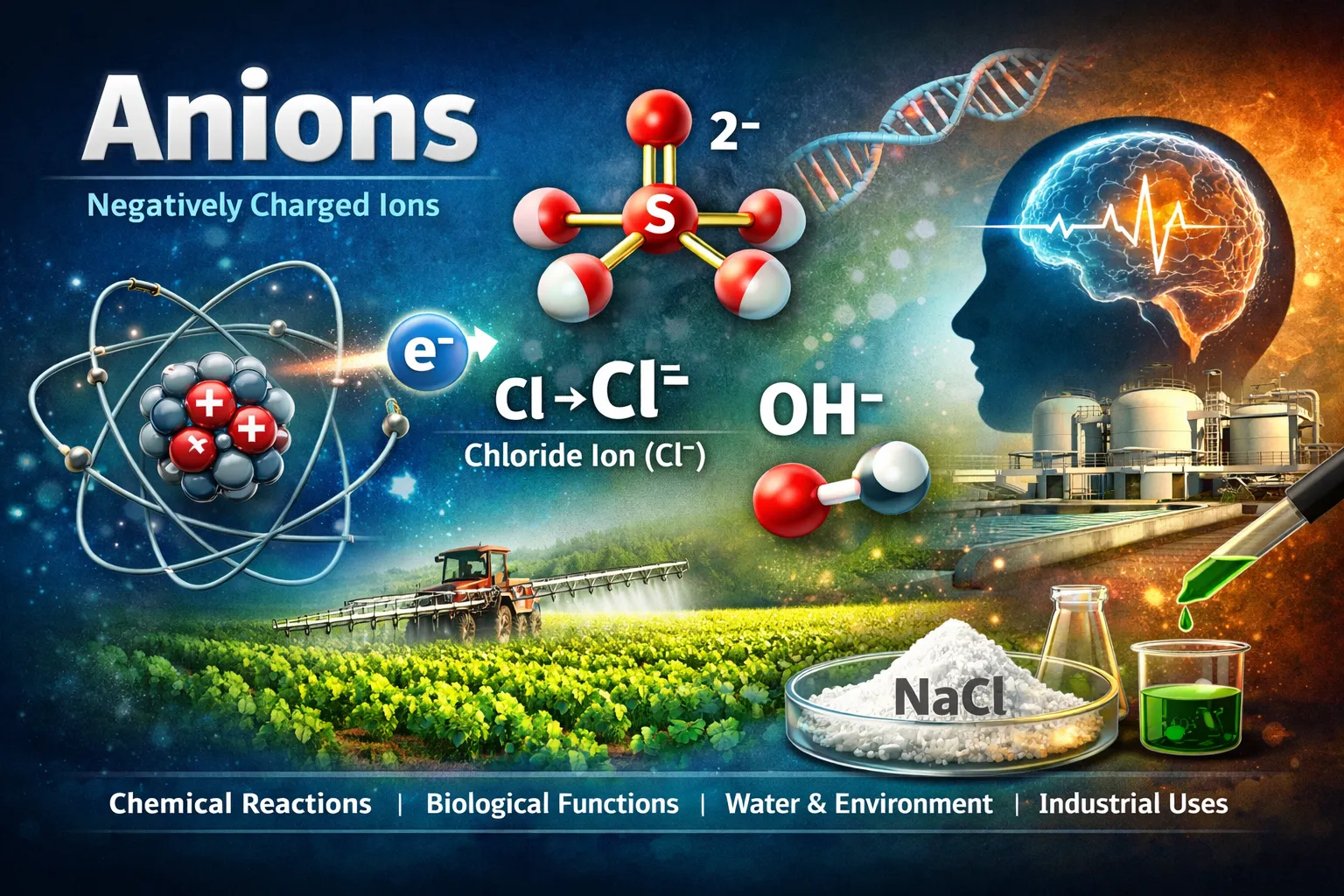
An anion is a negatively charged ion formed when an atom or molecule gains one or more electrons. These ions play a major role in chemistry, biology, environmental science, and even everyday processes like digestion and water purification. Understanding how an anion forms, its types, and how it behaves helps explain chemical reactions, electrolyte balance in the body, and many industrial applications.
What Is an Anion? (Simple Definition)
An anion is an atom or group of atoms that carries a negative electrical charge because it has gained extra electrons. Since electrons are negatively charged, gaining them gives the particle an overall negative charge.
Quick Facts About Anions
- Carry a negative charge
- Form by gaining electrons
- Usually created by nonmetal elements
- Important in chemical bonding and reactions
- Found in biological fluids and environmental systems
This simple concept is essential in understanding ionic compounds, electrolyte solutions, and chemical stability.
How Does an Anion Form?
An anion forms when an atom gains electrons to become more stable. Atoms naturally try to complete their outer electron shell, often referred to as achieving a stable electron configuration.
Step-by-Step Formation of an Anion
- An atom has an incomplete outer shell.
- It gains one or more electrons.
- The number of electrons becomes greater than protons.
- The atom develops a negative charge and becomes an anion.
Example
- Chlorine atom gains one electron.
- It becomes a chloride ion (Cl⁻).
- The added electron gives chlorine a stable outer shell.
This process commonly happens during ionic bonding, where one atom loses electrons while another gains them.
Common Examples of Anions
Many familiar chemical substances contain anions. Some are simple single-element ions, while others are made of multiple atoms.
Monatomic Anions (Single Atom)
These anions consist of only one atom.
- Chloride (Cl⁻) – Found in table salt and body fluids
- Oxide (O²⁻) – Plays a role in metal oxides
- Sulfide (S²⁻) – Present in certain minerals
- Fluoride (F⁻) – Used in dental care products
- Nitride (N³⁻) – Found in specialized compounds
Polyatomic Anions (Multiple Atoms)
These consist of two or more atoms bonded together.
- Sulfate (SO₄²⁻) – Found in detergents and fertilizers
- Nitrate (NO₃⁻) – Common in agriculture and explosives
- Carbonate (CO₃²⁻) – Present in limestone and baking soda
- Phosphate (PO₄³⁻) – Important for DNA and fertilizers
- Hydroxide (OH⁻) – Found in bases like soap
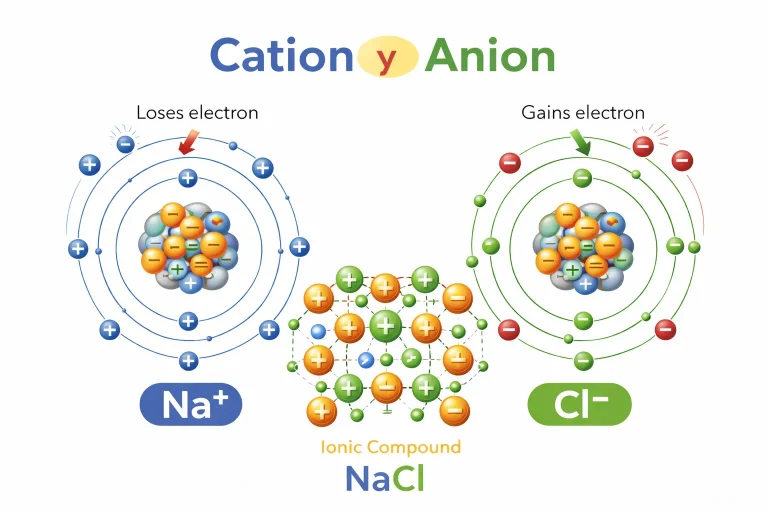
Difference Between Anion and Cation
Many people confuse an anion with a cation. The difference is simple but important.
| Feature | Anion | Cation |
|---|---|---|
| Charge | Negative | Positive |
| Electron Movement | Gains electrons | Loses electrons |
| Usually Formed By | Nonmetals | Metals |
| Example | Cl⁻, SO₄²⁻ | Na⁺, Ca²⁺ |
Remembering this difference helps in balancing chemical equations and understanding ionic compounds.
Why Are Anions Important in Chemistry?
Anions are essential because they participate in chemical reactions and help create stable compounds.
1. Formation of Ionic Compounds
Anions combine with positively charged ions to form ionic compounds. For example:
- Sodium ion (Na⁺) + Chloride ion (Cl⁻) = Sodium chloride (NaCl)
2. Chemical Reaction Balance
Anions help maintain charge balance during reactions, especially in solution chemistry.
3. Acid-Base Chemistry
Certain anions determine whether a substance behaves as an acid or base. For example:
- Hydroxide ions are responsible for alkaline solutions.
Role of Anions in the Human Body
Anions are not just limited to chemistry labs. They play critical roles in biological systems.
Electrolyte Balance
The human body depends on several anions to maintain fluid balance and nerve function.
Important biological anions include:
- Chloride ions help maintain osmotic pressure.
- Phosphate ions support energy transfer through ATP.
- Bicarbonate ions regulate blood pH.
Nerve Signal Transmission
Anions assist in transmitting electrical signals between nerve cells. These signals allow muscles to contract and the brain to communicate with the body.
Metabolism and Energy Production
Certain polyatomic anions support cellular respiration and metabolic reactions.
Properties of Anions
Understanding the physical and chemical properties of an anion helps predict its behavior.
Key Characteristics
Negative Electrical Charge
The defining property of an anion is its extra electrons, which create a negative charge.
Increased Atomic Size
When atoms gain electrons, electron repulsion increases, making the ion slightly larger than its neutral atom.
Attraction to Positive Ions
Anions naturally bond with positively charged ions due to electrostatic attraction.
Conductivity in Solution
When dissolved in water, anions contribute to electrical conductivity, which is why saltwater conducts electricity.
How Anions Behave in Chemical Bonding
Ionic Bonding
Anions commonly form ionic bonds with cations. This bond occurs due to electrostatic attraction between opposite charges.
Hydrogen Bonding
Certain polyatomic anions participate in hydrogen bonding, which affects biological molecule stability.
Covalent Interaction
Some anions exist within molecules where atoms share electrons instead of transferring them completely.
Anions in Environmental Science
Anions are critical in environmental monitoring and water quality analysis.
Water Treatment and Purification
Several anions are tested in drinking water because their concentration affects safety.
Examples include:
- Nitrate contamination linked to agricultural runoff
- Fluoride levels monitored for dental health
- Sulfate affecting water taste and corrosion
Soil Fertility and Agriculture
Farmers rely on anion-based nutrients such as nitrates and phosphates to improve crop growth.
Industrial Applications of Anions
Anions are widely used across multiple industries.
Manufacturing and Chemical Production
- Sulfate and nitrate anions are used in fertilizers.
- Chloride ions are essential in plastics manufacturing.
Pharmaceutical Industry
Many medicines contain anions that help maintain stability and absorption.
Food Processing
Certain anions act as preservatives, flavor enhancers, and pH regulators.
How to Identify an Anion in Chemistry
Identifying an anion usually involves chemical testing or laboratory analysis.
Common Identification Methods
Flame Tests
Some anions influence the color of a flame during testing.
Precipitation Reactions
Mixing solutions can produce solid compounds that help identify specific anions.
Spectroscopy Techniques
Advanced methods analyze light absorption patterns to determine ion presence.
Frequently Asked Questions About Anions
What is the easiest way to remember an anion?
An anion carries a negative charge because it gains electrons. The extra electrons give it the negative sign.
Are anions always harmful?
No. Many anions are essential for life, including chloride and phosphate ions.
Do anions exist in everyday substances?
Yes. Table salt, baking soda, toothpaste, and fertilizers all contain important anions.
Can anions conduct electricity?
Yes. When dissolved in water, anions help carry electrical current.
Real-Life Examples of Anions Around You
Anions are part of daily life even if most people do not notice them.
- Toothpaste contains fluoride ions.
- Carbonated drinks include carbonate and bicarbonate ions.
- Household cleaning products often contain sulfate ions.
- Batteries rely on ion movement, including anions, to generate power.
Interesting Facts About Anions
- Anions are typically formed by elements on the right side of the periodic table.
- Many essential nutrients exist as polyatomic anions.
- Atmospheric chemistry involves anions that influence pollution and climate reactions.
- Biological cells carefully regulate anion concentration for survival.
Tips for Understanding Anions Easily
If learning about ions feels confusing, these tips can help:
- Remember: Anions are negative because they gain electrons.
- Associate nonmetals with anion formation.
- Practice balancing ionic compounds.
- Study common polyatomic ions frequently used in chemistry.
Conclusion
An anion is a negatively charged ion created when an atom or molecule gains electrons to achieve stability. These ions are essential for chemical bonding, biological functions, environmental systems, and industrial processes. From maintaining electrolyte balance in the human body to supporting agriculture and manufacturing, anions influence countless natural and technological processes.
Learning about anions helps build a strong foundation in chemistry and offers valuable insight into how substances interact at the molecular level. Their presence in daily products, environmental systems, and living organisms highlights their importance in both science and everyday life.
Strongest Metal in the World: Strength, Properties, and Real-Life Uses Explained